HSC Chemistry Notes
Module 6 / Inquiry Question 1
Agenda + Quick Links
Overview of Week 5 Inquiry Question – What is an acid and a base?
Learning Objective #1 – Investigate the correct IUPAC nomenclature and properties of common inorganic acids and bases
Learning Objective #2 – Prepare and use indicators to illustrate the characteristics and properties of acids and bases and their reversible reactions
Learning Objective #3 – Predict the products of acid reactions with bases, carbonates and metals
Learning Objective #4 – Investigate the applications of neutralisation reactions in everyday life and industrial processes
Learning Objective #5 – Explore changes in the definitions of acid and base over time, including Arrhenius and Bronsted-Lowry theories
Learning Objective #6 – Explore changes in the model of acids and base over time, including Arrhenius and Bronsted-Lowry theories
Learning Objective #7 – Measure the enthalpy of neutralisation reactions through an experiment
HSC Chemistry Lecture Video – Properties of Acid and Bases
Week 5 Homework Set (Essential for Band 5!)
Week 5 Curveball Question (Moving from Band 5 to Band 6!)
Week 5 Extension Questions
Solutions to Week 5 Homework Set
Overview of Week 5 Notes
In this week’s notes, we will cover multiple aspects of acid and base substances.
We have briefly talked about acids and bases in Module 5. Namely, Arrhenius’s definition of acids and bases as well as the Bronsted-Lowry definitions of acids and bases.
If you have forgotten about them, I recommend go back revisiting them in Module 5. However, even if you have forgotten about them, don’t worry. We will go over their definitions again during this week’s notes.
In addition to exploring the definitions of Arrhenius’s and Bronsted-Lowry’s definitions of acids and bases, we will also be looking at two more historical scientists’ definition of acids – Lavoisier and Davy. They did not talk about bases. Collectively, the works of these four scientists enhanced our understanding of acids and bases in modern chemistry studies.
However, before we look into the historical development of the theories of acids and bases over time, I will through you into the deep end. We will first look at the method in which you name inorganic acids and bases when given their chemical formulas. The method in which you name classes of substances is called nomenclature.
We follow the IUPAC (International Union of Pure and Applied Chemistry) rules when performing naming substances.
Once we got the basics of naming inorganic acids and bases, we will move on to examine the properties of acids and bases through the use of indicators.
After examining the properties of acids and bases, we will move on to see how acids react with bases, carbonates and metals. We will see how these different chemical reactions will form different products.
Following this, we will examine the concept of neutralisation which is essentially an acid-base reaction (acid reacting with a base). We will examine how acid-base or neutralisation reactions are used in everyday life and in industry.
Finally, we will finish off this week’s notes by looking at how we can calculate the enthalpy change for neutralisation reactions.
Learning Objective #1 - Investigate the current IUPAC nomenclature and properties of common inorganic acids and bases
Although we have touched on the IUPAC naming methodology for inorganic acids and bases in the Preliminary HSC course, we will go over this again here as it is a specific learning objective.
Notice that the learning objective requires us to learn how to name inorganic acids rather than organic acids.
We will deal with the nomenclature of organic compounds in Module 7!
Organic acids contains carbon atoms whereas inorganic acids generally do not. There are always exceptions to everything in the world. But, trust me, you will be okay.
Let’s continue moving forward to learn the IUPAC rules for naming inorganic acids and bases!
The first class or type of inorganic acid that we will explore is called binary acid. These are acids that contain hydrogen bonding with a non-metal.
Type of inorganic acid: Binary acids
These are acids that that contain hydrogen that are bonded with a non-metal element.
NOTE: You won’t find oxygen atoms in binary acids. This is because that’s a different type of acids (oxyacids) which we will explore later.
A common example of a binary acid that you have came across is hydrochloric acid, HCl.
To name binary acid, it is simple. Here are the steps.
Step 1: Name the hydrogen first. You do this simplifying the word to just ‘hydro’.
Step 2: Find the root name of the non-metal.
You vocabulary list of root names of non-metals will grow as you gain exposure to them.
We have attached a list below of the root names for common non-metals that you need to know when naming inorganic acids for the HSC Chemistry course.
Step 3: Combine ‘hydro’ and the root name of the non-metal. Start with hydro first, followed by the root name.
Step 4: Combine the hydro + rootname with ‘ic’
Step 5: Following the word you generated at the end of step 4, write ‘acid‘ after leaving a space between the two words.
List of root names for common non-metals (& transition metal)
Nitrogen (N) – Nitr
Phosphorus (P) – Phosph
Oxygen (O) – Ox
Iodine (I) – Iod
Fluorine (F) – Fluor
Chlorine (Cl) – Chlor
Bromine (Br) – Brom
Sulfur (S) – Sulf
Hydrogen (H) – Hydr
Carbon (C) – carb
Transition metal – Chromium (Cr) – Chrom
Transition metal – Manganese (Mn) – Mangan
Practical example of naming a Binary Acid
Let’s do an example. How do you name the HCl compound?
Step 1: ‘Hydro’
Step 2: The non-metal here is chloride. The root name for chloride is ‘chloro’
Step 3: Combine hydro and the root name. This gives ‘hydrochloro’
Step 4: Combine the root name with ‘ic’. This gives ‘hydrochloric’
Step 5: Add the word ‘acid’ following the name of the inorganic acid. This gives ‘hydrochloric acid’.
Hence, the answer to naming the compound, HCl, is hydrochloric acid.
Type of inorganic acid: Oxyacids
Another type of inorganic acid is oxyacids. Sometimes, you may see this type of acid being referred to as oxoacids.
Unlike binary acids, oxyacids contain oxygen atom(s), hence the term ‘oxy’ in their name.
In terms of common features shared between oxoacids and binary acids, both types have a hydrogen and non-metal component to them.
So, let’s try name them! Below are the steps.
Step 1: Ignore the hydrogen. This means that we don’t need to name it, aka, don’t write hydro.
Step 2: Find the root name of the non-metal group. Sometimes, instead of a non-metal, there may be a transitional metal instead. Such as chromium or manganese.
Step 3: Combine ‘ous’ or ‘ic’ with the rootname. Use ‘ous’ if the non-metal is in lower oxidation state. Use ‘ic’ if the non-metal is in the higher oxidation state. Generally, acids with ‘ic’ are more common than those with ‘ous’.
Step 4: Write acid following the acid’s name.
Practical example of naming an Oxyacid
Let’s apply the four steps above to try name the oxyacid, HNO3.
Step 1: We ignore the hydrogen.
Step 2: The root name of the non-metal, nitrogen, is ‘nitr’
Step 3: The oxidation state of nitrogen here, in HNO3, is +5.* The alternative is HNO2 where nitrogen has an oxidation state of 3+. You may know that nitric acid is the more common acid amongst the two based on frequency of encountering them. For these two reasons, we select to use ‘ic’. This gives us ‘Nitric’, the one where the central nitrogen atom higher oxidation state.
NOTE: We learnt about oxidation states in Preliminary HSC Chemistry Course.
Step 4: We add ‘acid’ to ‘nitric’. This gives us nitric acid.
So, our answer in naming the oxyacid, HNO3, is nitric acid.
Exceptions for the nomenclature of oxyacids
For oxyacids that have the phosphorus or sulfur non-metals, we need to modify their root names after Step 4.
For oxyacids containing phosphate central atom, we need to add ‘or‘ after its root name.
For oxyacids that have sulfur as the central atom, we need to add ‘ur‘ after its root name.
This means that INSTEAD OF:
H3PO4 = Phosphic acid, we call it phosphoric acid instead.
That is, we added ‘or’ after phosphorus root name, phosph.
H2SO4 = Sulfic acid, we call it sulfuric acid instead.
That is, we added ‘ur’ after sulfur’s root name, sulf.
Prefixes for oxyacids
Sometimes, you may encounter the need to use prefixes when naming oxyacids. However, in most cases, these oxyacids with prefixes are incorporated in a question without requiring you to name them.
So, either way, it is good to understand how the acids looks like if they carry a prefix in front of their name.
You will see two possible prefixes that is incorporated in front the name of oxyacids.
These prefixes are ‘hypo‘ and ‘per‘.
By far, the most common oxyacid used in HSC Chemistry questions that have both of those prefixes are the oxyacid containing the chlorine atom.
Previously, we touched on the fact that we can have ‘ous‘ or ‘ic‘ after the root name of the non-metal based on its oxidation state. The one with the higher oxidation state have ‘ic’ after its root name whereas the other have ‘ous’.
HClO2 = chlorous acid (oxidation state of chlorine atom is 3+)
HClO3 = chloric acid (oxidation state of chlorine atom 5+)
We also know that the one with the higher oxidation state (+5) is the one that we encounter the most, i.e. the more common oxyacid out of all the oxyacids that contains chlorine atom.
If we remove an oxygen from chlorous acid, so that it becomes HClO, we need to add the prefix ‘hypo‘ in front of chlorous acid. Oxidation state of chlorine decreased from 3+ to 1+.
So, HClO is called hypochlorous acid.
If we add an oxygen atom to chloric acid, so that it becomes HClO4, we add the prefix ‘per‘ in front of chloric acid. Oxidation state of chlorine increased from 5+ to 7+.
So, HClO4 is called perchloric acid.
Naming Inorganic Bases
Typically, you will see bases either written with a metal or ammonium ion to begin with. This is then followed by a hydroxide ion.
In some cases, an oxide may be present rather than hydroxide ion. We will touch on oxides later.
So, to name an inorganic base, here are the steps.
Step #1: Name the metal or ammonium ion first, depending on the compound whether it has an ammonium ion or not.
Step #2: Add the word ‘hydroxide’ following the name of the metal or ammonium ion.
Example of naming inorganic base
Let’s try name the base, Mg(OH)2.
Step #1: The name of the metal in the compound is magnesium, Mg.
Step #2: We add the word ‘hydroxide’ after magnesium.
So, the name of the base, Mg(OH)2, is magnesium hydroxide.
Other forms of acids and bases: Metallic and Non-Metallic Oxides
Do note the following:
Metallic oxides are basic because they can dissolve in water to form basic solutions.
Example of metallic oxide: Calcium Oxide, CaO
Non-Metallic oxides are acidic because they dissolve in water to form acidic solutions.
Example: of non-metallic oxide: Carbon Dioxide, CO2
So how do you name these acids and bases? This was taught in preliminary HSC chemistry course. But we will quickly go through it again because we can o.o.
So, we first name the metallic or non-metallic by stating the name of the element that is located more towards the left of the periodic table first. Take the metallic oxide, CaO as an example.
In our example of CaO, calcium is written in the chemical formula first as well as named first because it is situated more towards the left of the periodic table than oxygen.
Next, we write the root name of the second element in the compound. For oxygen, the root name is ox.
Following this, we then add ‘ide‘ to the root name of the second element. So in our example, it will become ‘oxide’.
After that, we add prefixes to the name of the first element and second element depending on the number of atoms it has.
For example, N2O4 is called Dinitrogen tetroxide. ‘Di‘ prefix is used because there are two nitrogen atoms in a N2O4 compound and the ‘tetra‘* prefix is used because each N2O4 compound has four oxygen atoms.
* Recall from Week 1 HSC Preliminary Notes: The prefix ‘tetra’ is shorten to ‘tetr’ if the prefix end with ‘a’ or ‘o’ AND the root name starts with ‘a’ or ‘o’.
Here, tetra ends with ‘a’ and the root name ‘ox’ (for oxygen) starts with ‘o’.
Hence, we cut off the last letter in the prefix ‘tetra’. Recall that this applies to other prefixes like penta, hexa, etc.
If you are not familiar with the use of prefixes here, revisit Week 1 Notes in Preliminary HSC Chemistry Course available on this website. We went through the use of prefixes under ‘naming covalent compounds’.
Learning Objective #2: Conduct an investigation to demonstrate the preparation and use of indicators as illustrators of the characteristics and properties of acids and bases and their reversible reactions
What are indicators?
An indicator is a substance that changes colour as the pH of a solution changes.
pH is unitless value that is assigned to solutions in order to compare the relative acidity between solutions.
If you wish to learn about why indicators changes colour when pH of a solution changes, feel free to research about Ostwald’s theory as well as Quinonoid’s theory.
However, we will attempt to briefly explain the colour change using an equilibrium equation and Le Chatelier’s Principle in this learning objective.
NOTE: We will encounter another measurement term as a pOH which is a measure of basicity.
pH values can range from 0 to 14. You may already know that:
Acidic solutions have a pH less than 7.
Neutral solutions have a pH equal to 7.
Basic solutions have a pH more than 7.
NOTE: pH values can exceed 14 or be less than 0 (negative), if the concentration of the strong acid or base is very high. We will explore what is meant by strong acid/base and concentrated strong/bases next week.
Indicators are used because their colour changes are useful in assisting you in determining the approximate pH of a substance such as a solution. They are also useful to compare the relative acidity between different solutions.
Indicators are usually weak acids or bases, meaning they form an equilibrium in solution. In most cases, the indicator molecule reacts with water.
H2In(aq) + H2O(l) <-> 2H3O+(aq) + In2- (aq)
NOTE: In some cases, the indicator can have a valency of 1–.
H2In will have a unique colour compared to In2-.
Note that ‘ln’ is short for indicator.
‘H2ln’ just means that the indicator has is bonded to two hydrogen atoms.
If an acid is present or added into solution, this would mean that the equilibrium position for the above equilibrium reaction will shift to the left in order to minimise the increase in [H+] according to Le Chatelier’s Principle. This would mean the solution will develop a colour that H2In gives in solution.
Vice versa, if a base is present or added in solution, the equilibrium position will shift to the right as the concentration of H3O+ ions will decrease as it reacts with the hydroxide ion from the base to produce water.
Hence, the shift of the equilibrium position to the right will give the unique colour In2- which you observe as a colour change.
This is the reason why you see colour change when doing your titration experiments, which we will explore later in Week 7’s Notes.
Some indicators changes colour over a small pH range whereas other indicators changes colour over a large pH range.
Let’s have a look at and compare two indicators that changes colour over different pH ranges.
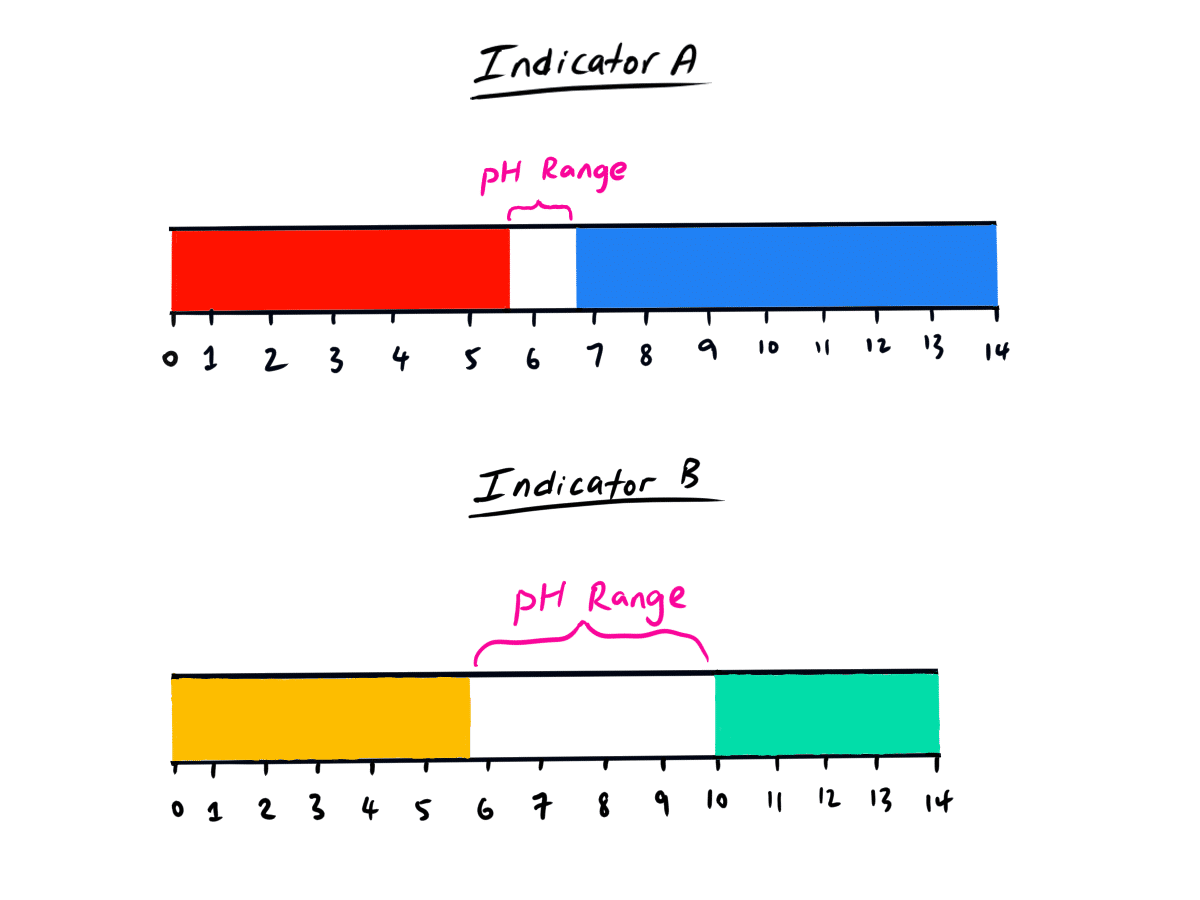
In the diagram below, Indicator A changes colour over a small pH range whereas Indicator B changes colour over a small pH range.
Which indicator is more accurate?
Well, since indicator A changes colour (from red to blue and vice versa) over a smaller pH range than indicator B. It is a more accurate and more effective* indicator as, from the indicator’s colour change, you will be able to gather that substance you are testing the pH for will have a pH of 5.5 – 6.5. (thanks to the indicator)
Since indicator B changes colour over a larger pH range (from 5.5 to 10) than indicator A, it is a less effective* and less accurate indicator than indicator A. This is because it is harder for you to pinpoint the exact pH of your substance that you want to measure the pH value for by observing the indicator colour.
That is, the pH of the substance that you are using the indicator to measure would be less certain due to large pH range over which the indicator changes colour.
* NOTE: The term ‘effective‘ for indicators depends on the purpose of using the indicator. If you just want to see if the substance is acidic or basic and nothing more specific, you can simply use an indicator that changes colour over the pH range between 7 to less than 7 to see if the substance is acidic. Alternatively, you can also use an indicator that changes colour over the pH range from 7 to greater than 7 to see if the substance is basic.
Another method of determining pH is using a litmus paper to easily distinguish if the substance is acidic or basic.
If the substance is neutral, then if you test the substance (solution) using red and blue litmus paper, both litmus papers will not change colour.
If the substance is acidic and you dip a blue litmus paper into the solution containing the substance, the paper will turn red.
Vice versa, if the substance is basic and a red litmus paper is used, the paper will turn to a blue colour.
If you wish to use the indicator (A and B) to pinpoint the exact pH of the substance, then indicator A is more effective in doing the job than indicator B. However, indicator A still fails to pinpoint the pH exact as the substance can have a pH of anywhere from 5.5 to 6.5.
To pinpoint the exact pH, a pH probe can be used. It is more accurate than indicators.
Preparation of indicators - Red Cabbage Extract
Let’s prepare an indicator!
The indicator that we will be preparing here is a natural indicator. It is one that you will be likely preparing at school too.
Method
Step 1: Cut the red cabbage leaves into fines pieces using a knife and cutting board.
Step 2: Put the cabbage leaves into an empty 500ml beaker until the leaves reach a height of approx. 2cm.
Step 3: Fill the beaker with distilled water to cover the cabbage and gently boil the mixture. Stir the mixture when boiling using a stirring rod to facilitate uniform boiling.
Step 4: Decant the cabbage extract into a separate 500ml beaker and document the extract’s colour. Dispose the cabbage leaves into a bin.
Step 5: Pour equal quantities of cabbage extract into six test tubes. Number the test tubes from 1 to 6 and record their initial colours.
Step 6: Add 20mL of distilled water into test 1 and 0.01M of HCl, acetic acid, ammonia and sodium hydroxide in test tubes 2, 3, 4 and 5 respectively. Test tube 6 is used as a control with only red cabbage extract.
Step 7: Swirl the test tube and record any colour changes in each test tubes.
Step 8: Repeat step 5 and 6 to test the reliability of indicator and results.
Expected results
The colour of the red cabbage extract should be purple or dark red in a neutral environment such as when no substance is added to the indicator.
When an acidic is added to the indicator, the indicator’s colour should change from purple to pink (red at very low pH, i.e. 1 or less than 1).
When a base is added to the indicator, the indicator’s colour should change from purple to green (or yellow at very high pH, i.e. 13-14)
When a neutral solution such as distilled water is added to the indicator, the red cabbage indicator will not change colour and remain purple.
Within the pH range, the indicator’s colour should be purple.
Risk assessment
Although the ammonia solution is dilute, it still releases toxic fumes which you should avoid inhaling. Use the fume cupboard to reduce exposure to toxic fumes.
HCl is corrosive and NaOH is caustic. If any acid or base makes contact with your skin, wash your hands immediately and thoroughly.
Wear safety googles at all times to avoid any accidental splashes of acids and bases from reaching the eye.
Improvements
A pH probe can be used to determine the precise pH of the solutions where a calibrating solution is used to calibrate the pH probe to improve accuracy and reliability of results. The calibrating solution is a system that is known as a buffer. We will talk about buffers in next week’s notes.
Alternative Method
Step 1 – 4: The same as previous method.
Step 5: Using 1M HCl and 1M of NaOH solutions, dilute the solutions using distilled water such that a set of solutions with different, known pH values are created (1-14) in 14 separate test tubes. Then compare colour of indicator to different the acid and base solutions with different pH values created through dilution.
Properties and characteristics of acid
Some of the common properties that you may see from acids are:
Turning a blue litmus paper red. Litmus paper is a natural indicator.
Tastes sour.
Conductors of electricity when dissolved in water, due to dissociation of acid molecules into their constituent ions, giving rise to free ions.
Dissolves limestone (calcium carbonate) or other carbonates, producing carbon dioxide gas as a product
Reacts with reactive metals and produces hydrogen gas as a product.
Does not react with lipids or fats.
Properties and characteristics of bases
Some of the common properties that you may see from bases are:
Turning a red litmus paper blue. Litmus paper is a natural indicator.
Tastes bitter.
Akalis (soluble base) will conduct electricity when dissolved in water, due to dissociation of acid molecules into their constituent ions, giving rise to free ions.
Does not react with limestone or reactive metals.
Reacts with lipids or fats over time.
Possesses a soapy texture when handled physically (which you shouldn’t do). Commercial soap products contain bases.
Reversible reactions of indicators in solution
Suppose you have the following indicator dissolved in a beaker of water. This indicator can exhibit two colours which are red and yellow.
Equation 1: H2In(aq) + H2O(l) <-> 2H3O+(aq) + In2- (aq)
H2ln colour = Red
ln2- colour = Yellow
Note that ‘ln’ is short for indicator.
‘H2ln’ just means that the indicator has is bonded to two hydrogen atoms.
Suppose that equation one proceeds to equilibrium when you dissolve the indicator in the solution containing the test solution or you wish to add the test solution into the indicator.
Either way, IF the test solution contains hydronium ions (e.g. adding acid to the beaker of water or the test solution already contains hydrogen ions when indicator was added), it will shift the equilibrium position of equation one to the left according to Le Chatelier’s Principle to minimise the increase in hydronium ion concentration. This would cause the solution change colour from yellow to red.
If the solution contains hydroxide ions, the hydronium ions formed from the dissociation of the indicator molecule will react with the hydroxide ion to form water.
This decreases the concentration of hydronium ions in solution and thus will cause the equilibrium position of equation one to shift to the right as per Le Chatelier’s Principle to minimise the decrease in [H3O+]. This would cause the solution to change colour from red to yellow.
In short, we can see that the indicator would undergo a colour change using the equilibrium equation above.
Sometimes you may see this equation instead:
HIn(aq) + H2O (l) <-> H3O+(aq) + ln–(aq)
This just means that the indicator has a valency of -1. However, most indicators have a valency of -2 as shown above.
Learning Objective #3 - Predict the products of acid reactions and write balanced equations to present:
- Acids and bases
- Acids and carbonates
- Acids and metals
Acid and base reactions
When you react an acid with a base, you will get salt and water as products.
Acid + Base -> Salt + Water
The salt can be neutral, acidic or basic properties depending on the strength of the acid and base used in the reaction.
To determine the acidity or basicity of a salt, you can dissolve it in water.
If the salt give rise to a solution with a pH of less than 7, it is an acidic salt.
If the salt give rise to a solution that has a pH of more than 7, it is a basic salt.
If the resulting solution has a pH equal to 7, it is a neutral salt.
Suppose you have mix the following acid and base solutions.
Equation 1: HCl(aq) + NaOH(aq) -> NaCl(aq) + H2O(l)
Sodium chloride will be the salt in the above scenario. It is a neutral salt as it yields a pH of 7 when you dissolve the ionic compound in water.
Acid and base reactions are called neutralisation reactions if they involve hydrogen (or hydronium ions) reacting with hydroxide ions to produce water. This can be expressed as:
H+(aq) + OH–(aq) -> H2O(l)
This is why water is produced as a product in the equation involving the reaction of hydrochloric acid with sodium hydroxide.
NOTE: It does not really matter if you use hydrogen ions or hydronium ions. This is because most of the acid and solutions that you be handling is diluted with water.
For example, HCl + H2O -> H+(aq) + Cl-(aq) + H2O(l).
The hydrogen ions can then react with water to form hydronium ions. This can be expressed as:
HCl + H2O -> H3O+(aq) + Cl–(aq)
This is why pH can be expressed in two forms:
pH = -log10[H+] or
pH = -log10[H3O+]
In general:
A strong acid reacting with a strong base will give you a neutral salt (and water)
A strong acid reacting with a weak base will give you an acidic salt (and water)
A weak acid reacting with a strong base will give you a basic salt (and water)
Acids and carbonate reactions
Carbonates are substances that contain the carbonate polyatomic ion.
Some examples of carbonates include:
Calcium carbonate, CaCO3
Sodium carbonate, Na2CO3
Magnesium carbonate, MgCO3
Ammonium carbonate, (NH4)2CO3
When an acid reacts with a carbonate, you will get a salt, water and carbon dioxide gas as products.
Acid + Metal Carbonate -> Salt + Water + Carbon Dioxide
Usually in HSC, you will see an acid reacting with a metal carbonate or ammonium carbonate.
For example, suppose you have the reaction between HCl and calcium carbonate. You can express the reaction as follows:
HCl(aq) + CaCO3(s) -> CaCl2(aq) + CO2(g) + H2O(l)
Calcium chloride is formed as a salt. You will also see carbon dioxide bubbles as it evolves in the solution. Water will also be produced as a product.
If you have a metal hydrogen carbonate such as sodium hydrogen carbonate rather than sodium carbonate, the products produced are the same. That is, salt, carbon dioxide and water.
HCl(aq) + NaHCO3(s) -> NaCl(s) + CO2(g) + H2O(l)
Acid and metal reactions
Acids will react with metals IF the metal is capable of displacing the hydrogen ions from the solution. This would mean that the solid metal will become metal ions by donating electrons and the hydrogen ions will accept such electrons to become hydrogen gas. If the metal is not capable of displacing the hydrogen ions from solution, no reaction will occur.
If a successful displacement occurs in an acid-metal reaction, we can express the reaction as:
Acid + Metal -> Salt + Hydrogen Gas
Suppose you have the reaction between hydrochloric acid and magnesium metal which can be expressed as follows:
2HCl (aq) + Mg(s) -> MgCl2(aq) + H2(g).
Magnesium chloride will be the salt in this case. The magnesium metal will displace the hydrogen ions in solution to form hydrogen gas where the hydrogen ions are derived from the dissociation of hydrochloric acid.
HCl(aq) -> H+(aq) + Cl–(aq)
Each hydrogen ion will accept an electron donated from magnesium metal where two hydrogen atoms can bond to form hydrogen gas.
This means that, after displacement, the overall reaction will be: Mg(s) + 2H+(aq) -> Mg2+(aq) + H2(g)
In preliminary HSC Chemistry, you have learnt about the metal reactivity series.
Reactive metals are those that readily and quickly reacts with acids. This is why these reactive metals that is readily reacts with acid are positioned above hydrogen in the reactivity series table.
By losing electrons to form metal cations in solution, these metals can achieve a more stable state (octet).
As you learnt in preliminary HSC course, as you move down a group on the periodic table, the number of electrons of elements increases significantly. These electrons occupy in energy shells that are far away from the nucleus. As the number of energy shell increases, the effect of ‘shielding’ increases.
This means that the element can achieve a more stable state by losing those electrons that are loosely attracted to the nucleus in the high energy shells.
This means the metal is capable of donating electrons to hydrogen ions. This results in metal ions in solution and the displacing hydrogen ions into hydrogen gas.
Unreactive metals normally do not react with acids. These metals are already in their stable state. Hence, the donation of electrons from these unreactive metals is less common.
However, if the acid is strong enough reducing agent, a reaction may proceed. That is, the unreactive metal may react with the acid.
Learning Objective #4 - Investigate the applications of neutralisation reactions in everyday life and in industrial processes
Now that we understand that neutralisation reactions involves the reaction of acids and bases to produce water and a salt, let’s explore different types of neutralisation reactions that are happening around us!
Neutralisation reactions in everyday life
Consuming antacid tablets to reduce acid levels in stomach. The antacid tablets contain magnesium hydroxide bases which reacts with hydrochloric acid in your stomach.
Mg(OH)2(s) + H2O(l) -> MgCl2(aq) + 2H2O(l)
This effectively reduces the level of stomach acid in your stomach. This have the effect of relieving the upset feeling that you feel in your stomach or any heartburns (chest pain) that you may experience.
Baking soda is used in the process of baking cakes and contains sodium hydrogen carbonate. When it makes contact with the buttermilk in the cake, a neutralisation reaction takes place. This is because NaHCO3 in baking soda can act as base and buttermilk is an acid.
Similarly, sodium hydrogen carbonate can be used to neutralise ant stings which contain formic acid. Formic acid is corrosive and can burn the skin. By neutralising the formic acid, the pain on the skin can be reduced.
NaHCO3(aq) + CH2O2(aq) -> NaCHO2(aq) + H2O(l) + CO2(g)
Neutralisation reactions as part of industrial processes
Generally speaking, most non-metal oxides form acidic solutions when dissolved in water.
Vice versa, metal oxides form basic solutions when dissolved in water.
An example of an non-metal oxide is carbon dioxide.
An example of a metal oxide is calcium oxide.
Factories may dispose liquid waste that is often acidic. Calcium oxide (a base) are added to neutralise these acidic factory effluents before they are allowed to be disposed outside the factory as per government’s environmental regulation.
Some factories produces sulfur dioxide which is treated by neutralising it by calcium oxide (lime) before they are allowed to be released from the factory and into the atmosphere. Sulfur dioxide is acidic.
In this case, the acidic oxide (SO2) reacts with an base (CaO) to form salt and water. This is an acid-base reaction.
SO2(g) + CaO -> CaSO3(s)
NOTE: The above acid-base reaction did not produce water.
It is not necessary to have water as product in acid-base reactions. According to Arrhenius’s theory, water must be present as a product for neutralisation reactions. However, according to Bronsted Lowry’s definition of acid-base reactions between acid and bases, water is not a compulsory product.
The Bronsted-Lowry’s definition of neutralisation requires a conjugate acid and base to be produced.
We will explore in greater detail about the two scientists’ definitions of acids, bases and neutralisation reactions towards the end of this week’s notes!
Another acid-base reaction that does not produce water is the reaction between ammonia and hydrochloric acid to produce ammonium chloride.
Anyways, returning to the learning objective, a second example of a neutralisation reaction used in industrial processes is the production of fertilisers that contains ammonium sulfate, (NH4)2SO4(aq)
This is produced by reacting ammonia gas with sulfuric acid. Note that this is a multi-step process as you need to combine hydrogen and nitrogen gas to form ammonia. You also need to process the air to get hydrogen.
NH3(g) + H2SO4(aq) -> (NH4)2SO4 (aq)
Learning Objective #5 - Explore the changes in the definitions and models of acids and bases over time, accounting for their limitations
We have briefly touched on the definitions of acids and bases in Module 5.
Specifically, the definition of acids and bases defined by:
Svante Arrhenius
Johannes Bronsted and Thomas Lowry, the pair often referred to as Bronsted-Lowry
In this learning objective, we will dive deeper into those two scientists as well as few other scientists. The other chemists that provided their definitions of acids and bases include:
Antoine Lavoisier
Humphry Davy
With this introduction out of the way, let’s get practical and start examining, in chronological order, how each scientists defined acids and bases!
Antoine Lavoisier | Oxygen Theory of Acids
Lavoisier was the first scientist to provide a definition for an acid.
His definition of acid, based off his experiment observations, proposes that non-metal compounds that contains one or more oxygen atom will act as acids when dissolved in water.
This is true, as mentioned previously, most non-metal oxides will produce acidic solutions when dissolved in water. The example that we gave was sulfur oxide and carbon dioxide. Another one is nitrogen dioxide.
Lavoisier attributes the oxygen atom as the reason behind why non-metal oxides produces acidic solutions when dissolved in water. In fact, he was the scientist who came up with the name ‘oxygen’.
Limitations of Lavoisier’s theory:
Lavoisier did not account for why metal oxides produced basic solutions when dissolved in water and why not acidic. He only said that non-metal oxides produces acidic solutions when dissolved in water.
Lavoisier’s theory of acids also failed to account for why some acids are stronger than others.
Humphry Davy | Hydrogen Theory of Acids
When I first saw this scientists name I thought it read Humpty Dumpty. Gosh, I don’t know why. I do wonder if anyone can relate though. If you can, please let me know in the ConquerHSC discord community group!
Anyhow, Humphry davy was the scientist that performed electrolysis on a solution containing HCl that was created by reacting aqueous sodium chloride with sulfuric acid.
Electrolysis is essentially the process of supplying an electric current through an ionic solution so that this energy is enough to split compounds into its constituent elements.
Essentially, Davy electrolysed the HCl in the solution and found that hydrogen and chlorine gas was produced. Note that Davy did not know the acidic solution contained hydrochloric acid. He just knew that the solution was acidic. Because of Lavoisier’s definition of acidic solutions, Davy expected oxygen gas to appear. However, only hydrogen and chlorine gas was produced as a result of the electrolysis.
During Davy’s time, there were other experiments performed around the world. One of these included electrolysing acidic solutions such as HCN which also did not produce oxygen gas.
Due to these discoveries, Davy theorised that acids are substances that contains hydrogen atoms which allowed the acid to have their acidic properties. This is agreed upon by chemists today.
Limitations of Davy’s theory:
Davy did not account for why compounds, such as methane, which contains hydrogen atoms, were not acidic.
Davy failed to account for why some acids are stronger or weaker than other acids.
Davy’s theory cannot account for neutralisation reactions.
Davy’s theory cannot address the limitations in Lavoisier’s oxygen theory of acids. That is, why metal oxides produced acid solutions when dissolved in water and why they produced basic solutions.
NOTE: After Davy proposed his hydrogen theory of acids, a chemist by the name of Justus von Liebig refined Davy’s theory of acids. The modification was pertaining to how acids were substances that possessed a replaceable hydrogen atom. If the metal cannot replace the hydrogen of the acid, no reaction will occur. Therefore, in order for an acid and metal reactions can only occur, the acid must have a replaceable hydrogen.
As we have learnt previously,
Acid + Metal -> Salt + Hydrogen
Either way, this revised version of Davy’s theory of acid still had all the limitations stated above as part of Davy’s theory of acid. Also, he fails to account for why nitrogen dioxide, instead of hydrogen gas, is given off when nitric acid reacts with metals.
Svante Arrhenius | Hydrogen Ion Theory of Acids
We’re back to revisiting Arrhenius again!
Arrhenius researched and performed many experiments that involved electrolytes which involved ions.
Arrhenius proposed that all acids are substances that dissociate and produces hydrogen ions in solution when dissolved in water. He stated that it was the hydrogen ion is dissociated from the acid molecule that gave the substance (acid) its acidic properties.
Unlike Lavoisier’s and Davy’s theories, Arrhenius’s theory of acids accounts for the relative strength between different acids.
He stated that the strength of an acid is dependent on the degree of dissociation (which we now call degree of ionisation) where strong acids completely dissociate into its constituent ions when dissolved in water.
The emphasise on hydrogen ions provided the foundation for the pH scale and formula to be established, pH = log10[H+].
In terms of bases, Arrhenius proposed that all bases are substances that dissociate and produces hydroxide ions in solution when dissolved in water. He attributed the hydroxide ions in solution as the reason why the substance (base) has basic properties.
He stated that the strength of a base is dependent on the degree of dissociation where strong bases completely dissociate into its constituent ions when dissolved in water.
Through Arrhenius’s definitions of acids and bases he was able to account for why neutralisation occured when acids and base solutions are mixed together. He attributed the neutralisation reaction to the reaction between hydroxide ions with hydrogen ions to produce water as a product in solution. Since water is neutral (pH = 7), neutralisation reactions moves the overall solution towards 7.
NOTE the term ‘towards’ was used. This is because the resulting pH after neutralisation is not necessarily 7 as it would depend on the moles of hydrogen and hydroxide ions that are excess or not neutralised in solution.
Limitations of Arrhenius’s theory:
Arrhenius’s theory cannot be used to address Lavoisier’s and Davy’s theories of acid of why why metal oxides and metal carbonates produces basic solutions when dissolved in water. They do not have hydroxide ions when they dissociate in water. Many carbonates such as calcium carbonate is capable of neutralising with acids despite being insoluble. Arrhenius theory fails to account for this as his theory only deals with soluble bases (dissociating into ions) not insoluble bases which cannot dissociate into ions.
Arrhenius’s theory of acids and bases cannot account for why some salts, when dissolved in water, can produce basic solutions whilst others produce acidic solutions. It only accounts that salts formed as a result of acid-base reactions are neutral as the ions that they dissociate into in solution do not contain hydrogen and hydroxide ions. For example, sodium carbonate is acidic and calcium chloride is basic when dissolved in water.
Arrhenius theory of acids and bases does not consider the role of the solvent when an acid is dissolved in it or when a base is dissolved in it. It is the role of the solvent whereby a salt can be acidic or basic which is accounted for in the Bronsted-Lowry theory of acid and bases.
The solvent in which the acid and base is dissolved in plays a role in the degree of ionisation which Arrhenius fails to account.
Some acid-base reactions do not occur in solution where aqueous hydrogen and hydroxide ions react to form water. For example, ammonia gas reacting with hydrogen chloride gas to form solid ammonium chloride.
Theory does not explain the acid-base relationship between conjugate acid and conjugate base pairs in reversible reactions.
Johannes Bronsted - Thomas Lowry | Proton Donor Theory of Acids
The two scientists proposed a theory that helps address the limitations of Arrhenius’s theory of acids and bases.
The Bronsted-Lowry theory defines a substance as an acid if it acts as a proton donor.
The Bronsted-Lowry theory defines a substance as a base if it acts as a proton acceptor.
NOTE: A hydrogen ion is a proton. This is because a hydrogen ion has a charge of +1 which is formed after a hydrogen atom lost an electron. This is identical to a proton.
Similar to Arrhenius’s theory of acids and bases, the Bronsted-Lowry theory retains the importance of the hydrogen ions in explaining acid-base reactions. Bronsted-Lowry Theory is different to Arrhenius in that emphasise is placed on the exchange of protons or hydrogen ions in acid-base reactions rather than the dissociation of hydrogen ions and hydroxide ions.
This means that an acid molecule will NOT exhibit any acidic properties unless another substance (base) accepts the proton that is donated by the acid.
The concept of exchanging protons between acid and base molecules, rather than dissociation into aqueous ions (Arrhenius’s theory), means that Bronsted-Lowry’s theory can be applied to reactions to those that are not just occurring in aqueous solutions. For example, the acid and base can be in the gaseous state.
Unlike Arrhenius’s theory, the Bronsted Lowry takes into account the role of the solvent (e.g. water) in acid and base reactions. This means that the solvent is not inert and can act as an acid or base!
Here is an example where water (the solvent) is acting as a base:
HCl(g) + H2O (l) -> H3O+(aq) + Cl–(aq)
Bronsted-Lowry Acid = HCl
Bronsted-Lowry Base = Water
Conjugate Acid = H3O+
Conjugate Base = Cl–
NOTE: Every acid has its conjugate base. Vice versa, every base has its conjugate acid. Conjugate essentially means connected.
Notice that the conjugate acid can donate its proton to the conjugate base for the conjugate base to become the Bronsted-Lowry acid. By doing so, the conjugate acid can become the Bronsted-Lowry base.
The Bronsted-Lowry acid and base are always the reactants and the conjugate acid and base are always the products.
Here is an example where the water (the solvent) is acting as an acid:
NH3(g) + H2O(l) -> NH4+(aq) + OH–(aq)
Bronsted-Lowry Acid = H2O
Bronsted-Lowry Base = NH3
Conjugate Acid = NH4+
Conjugate Base = OH–
Limitations of Bronsted-Lowry Theory:
Cannot explain reactions between acidic and basic oxides
Cannot account for the nature of amphoteric substances that do not have protons. For example, AlCl3 and FeCl3 are acidic substances but cannot donate H+ ions
Cannot account for the nature of acid-base involving the exchange of protons when the solvent cannot donate or accept H+ ions (aprotic solvents). For example, benzene solvents are very stable and have very low tendencies to donate H+ or accept H+ ions. Other examples of aprotic solvents includes CCl4, chloroform, etc.
Learning Objective #6 - Conduct a practical investigation to measure the enthalpy of neutralisation
We need to design an experiment to determine the change in enthalpy of neutralisation between an acid and a base to form a salt and water.
We will be determining the standard enthalpy of neutralisation, that is, the experiment should be performed under standard conditions.
Standard conditions is referring to the surrounding conditions being 25 degrees celsius and 1 atmospheric pressure or 100 kilopascal (kPa).
Under these conditions, we measure change in enthalpy of neutralisation where exactly 1 mole of water is formed.
You may ask why don’t we measure standard enthalpy of neutralisation whereby 1 mole of salt that’s formed instead of water?
Well, the salt will just be spectator ions in solution.
For example, suppose there is a neutralisation between sodium hydroxide and hydrochloric acid:
NaOH(aq) + HCl(aq) -> NaCl(aq) + H2O(l), if we break this full equation into an ionic equation, we get:
Na+ (aq) + OH– (aq) + H+(aq) + Cl– (aq) -> Na+ (aq) + Cl– (aq) + H2O(l)
Do note that all these reactants and products are mixed together in the same solution or system.
If we removing the spectator ions from both sides, we get:
H+(aq) + OH– (aq) -> H2O (l)
This is why we tend to define standard enthalpy of neutralisation as the reaction of an acid and base under standard conditions (25 degrees celsius and 1 atmospheric pressure) to produce one mole of water.
The formula that we will be using to determine the change in enthalpy in a neutralisation reaction is:
ΔHneutralisation = – m x c x ΔT
m is the total mass of acid and base combined, measured in grams
c is the specific heat capacity of water, measured in joules/gram/kelvin (J/g/K)
ΔT is the change in temperature (Final temperature after reaction – Initial temperature before reaction), measured in kelvin
ΔHneutralisation is the changed in enthalpy, measured in kilojoules (hence since your specific heat capacity of water is in joules, you need to divide your answer by 1000 to convert into kilojoules)
NOTE: You may see ΔH written as Q or ΔQ, They mean the same thing for HSC calorimetry questions. At university, you will learn their difference.
NOTE: Capital “C” stands for heat capacity and lower case “c” stands for specific heat capacity.
Csubstance = msubstance x csubstance (i.e. heat capacity of substance = mass of substance multiplied by the specific heat capacity of that particular substance).
In some questions, you may be given sufficient information so you can take into the heat absorbed by the calorimeter. In those case, the ΔH formula is slightly more complicated and you need to use:
ΔHneutralisation = – [ (msolution x csolution) + (mcalorimeter x ccalorimeter) ] x ΔT
We can simplify the above equation to the following expression:
ΔHneutralisation = – [ (msolution x csolution) + (ccalorimeter) ] x ΔT
NOTE: The mass of the calorimeter is a fixed value or a constant. So, we ignore it in our calculation. Compared to the mass of the calorimeter, the mass of the solution can however change depending on the experiment. However, the mass of the calorimeter will always be fixed regardless if you use it or not.
As we have mentioned before, heat capacity (C) = mass x specific heat capacity.
So, the above formula can be simplified to:
ΔHneutralisation = – [ (Csolution + Ccalorimeter) ] x ΔT or
ΔHneutralisation = – [ (Csystem) ] x ΔT
NOTE: The mass of salt solution will be 1mL = 1 gram if we assume the salt solution has a density similar to water. As the salt solution will be comprised of mainly water, it can be justified that we assume that it consists of mainly water. So, it has a density of water = 1ml ~ 1gram. However, in some questions, you may be given the density of the solution. In those cases, so use the density of the solution that they give you in the question and use that to calculate the mass of the salt solution.
NOTE: You need to divide ΔH by the number of moles of water.
So, back to the experiment, it is perhaps useful for you to know that the neutralisation reaction between the acid and base will take place inside a calorimeter (copper or aluminium can). At school, you may use a polystyrene cup instead which is a good heat insulator.
Let’s explore an example of a neutralisation reaction between hydrochloric acid and sodium hydroxide and try measure the reaction’s enthalpy of neutralisation.
Procedure:
Step 1: Measure the mass of the calorimeter (or weight of calorimeter for the physics students :P).
Step 2: Set up calorimeter with temperature probe, stirring magnet and magnet stirrer.
Step 3: Measure 50ml of diluted 1M HCl and 50ml of diluted 1M NaOH solutions using a measuring cylinder.
Step 4: Turn on magnet stirrer and add the HCl into the calorimeter, ensuring that temperature probe is submerged in the acid solution.
Step 5: Record temperature for three minutes (there should be minimal change in temperature).
Step 6: Pour, all at once, the NaOH solution into the calorimeter, containing the HCl solution.
Step 7: Record temperature for three minutes (you should notice a spike in temperature the moment you add the NaOH as neutralisation reaction occurring which is exothermic).
NOTE: If you double the concentration of HCl, the increase in temperature should be twice as much. Assuming the [NaOH] you use remains constant.
Step 8: Calculate the molar heat of neutralisation (ΔHmol). Since this is molar change in enthalpy, the units will be in kJ/mol.
If you recall the objective of this learning objective, the question may ask you to calculate the molar heat of neutralisation. In that case, you need to divide ΔH by the moles of water. This is because standard enthalpy of neutralisation is defined by acid and base reacting to produce one mole of water. Hence, the change in standard enthalpy for neutralisation value will based off one mole of water.
To calculate the moles of water released, you need to identify the limiting reagent (acid or base) and apply mole ratio.
All neutralisation reactions are exothermic and so acid-base reactions will give a negative ΔH or change in enthalpy. You should be expecting a negative value. It is a way to check your answer.
NOTE: If the question asks you to determine the change in enthalpy of HCl (acid), you need to divide ΔH by the number of moles of HCl. Vice versa, if the question asks you to determine the change in enthalpy for the base, you need to divide ΔH by the number moles of the base.
NOTE: Since the acid was added into the calorimeter initially and then the base was added later, a question based off the procedure above will only ask you about the change in enthalpy for the acid. This is because you know the initial temperature of the acid as you submerged the temperature probe as per step 4. You do not know the initial temperature of the base (it was not part of the procedure). Due to this, you cannot calculate the molar change in enthalpy for the base. IF, however, you want to calculate the molar enthalpy for the base, you will need to perform an extra step. That is, you need measure and record initial temperature of the base like you did with the acid.
NOTE: The value of the change in enthalpy of reaction is equal to the value of the change in enthalpy of calorimeter. The only difference is that they are opposite in signs, i.e. ΔHreaction = – ΔHcalorimeter.
Errors to the deviation in your experimental value of the change in enthalpy of neutralisation from actual or published value
There are many reasons to why your experimental value of the change in enthalpy of neutralisation deviated from the published value.
Here could be some reasons why:
You assumed that the solution formed in the acid-base reaction consists of mainly water and so it would have the same specific heat capacity as water. In reality, the solution formed in reaction is not pure water.
You assumed that any heat that evolved from the reaction retained in the system and did not escape from the calorimeter. In reality, convention currents can carry away some heat that was formed as a result of neutralisation. You can use a draught shield to radiate any heat escaped from the calorimeter back towards the can.
You also assumed that all the thermal energy produced as a result of the neutralisation reaction was transferred to the solution. This means that you have to also assume that the calorimeter and temperature probe did not absorb any of the heat that was formed as a result of the neutralisation reaction.
Week 5 Homework Problem Set (Essential for Band 5)
Homework Question #1: Suppose you have 100ml of 0.5mol of sulfuric acid and 200ml of 1mol of KOH. You have both of them reacting in a bomb calorimeter. You measured the temperature have risen by 3.5 degrees celsius. Calculate the enthalpy change of neutralisation.
Homework Question #2: Define the standard enthalpy change due to neutralisation THEN explain why does the value of enthalpy change due to neutralisation involving any given strong acid reacting with a strong base is the same (always about 57kJ/mol)?
Homework Question #3: What does the value of standard enthalpy of the following reactions mean?
HCl(aq) + NaOH(aq) -> NaCl(aq) + H2O(l) ; ΔH = – 57.0 kJ/mol
CaO(s) + H2O(l) -> Ca(OH)2(s) ; ΔH = – 63.5 kJ/mol
Homework Question #4: You have a reaction between 200mL of 1.5M HCl and 200mL of 2M NaOH occurring inside a calorimeter. You recorded that the change in temperature is 14.2 degrees celsius. You also know that the specific heat capacity of the calorimeter to be 50 J/K. Calculate the molar enthalpy change due to the neutralisation reaction.
Homework Question #5: Assess by providing possible reasons to why your experimental value of the molar enthalpy change of strong acid and strong base neutralisation reaction is different from the published value.
Homework Question #6: Outline definitions of acids and bases presented by the scientists, including Lavoisier, Davy, Arrhenius and Bronsted-Lowry.
Homework Question #7: Describe the significance and limitations and of the definitions of acids and bases presented by the scientists, including Lavoisier, Davy, Arrhenius and Bronsted-Lowry.
Homework Question #8: Explain the advantages of two named neutralisation reactions used in everyday life.
Homework Question #9: Explain the advantages of two named neutralisation reaction used in industrial processes.
Homework Question #10: Define term ‘calorimetry’ and ‘calorimeter’
Homework Question #11: What is the definition of specific heat capacity of a substance and how does its definition differ from heat capacity of a substance?
Homework Question #12: Describe the experimental procedure which you use to determine the change in enthalpy due to neutralisation between HCl and NaOH
Homework Question #13: What is an indicator? Describe how would you prepare a natural indicator.
Homework Question #14: Explain the factors you would use to assess the accuracy AND effectiveness of an indicator? HINT: Typically, but not always, indicator’s accuracy refers to its pH range over which it changes colour.
Homework Question #15: Construct models to represent the theories of acids and bases proposed by Lavoisier, Davy, Arrhenius and Bronsted-Lowry. HINT: Use a chemical reaction that is relevant to the scientist’s theory of acids and bases and write out the appropriate chemical equation for that reaction you have selected. Use arrows to illustrate your point such as using arrows to show how the an acid molecule in your chemical equation is donating a proton and a base molecule accepting a proton.
True/False: Neutralisation reactions have positive molar change in enthalpy which results in the temperature of the salt solution in the calorimeter to increase.
True/False: Neutralisation reactions always result in the formation of water molecules.
True/False: Neutralisation reactions is an example of a proton transfer reaction.
Week 5 Curveball Question (Moving from Band 5 to Band 6!)
Curveball Question #1 – Suppose that you heat and dissolve 1 gram of glucose in a calorimeter, causing the temperature to increase from 25.2 degrees celsius to 29.4 degrees celsius. You were given information that the calorimeter has a specific heat capacity of 4.90 kJ/Celsius. Calculate the heat of combustion (i.e. enthalpy or change in enthalpy for the reaction, ΔH) for glucose, C6H12O6.
Curveball Question #2 – Suppose that you have 100ml of hydrochloric acid at concentration of 1M. You used it to neutralise 100ml of sodium hydroxide at with a same concentration of 1M. Calculate the increase in temperature. Suppose that the enthalpy change due to neutralisation is 57.3 kJ/mol.
Curveball Question #3 – Suppose you have determined the molar enthalpy change due to neutralisation to be 55.84 kJ/mol. The reaction was between HCl and NaOH. You know that you added 50.0 ml of sodium hydroxide with concentration equal to 1.05M and the volume of HCl you added was half the amount of sodium hydroxide at a concentration of 1.8M. Calculate the the final temperature. Assume that you measured the initial temperature of HCl and NaOH to be both exactly 27.47 degrees celsius. You are also given that the specific heat capacity of the solution to be 3.89 J/g/K and density of 1.05 grams per millimetre.
HINT: Remember to convert all temperature units to Kelvins! This is because your specific heat capacity is in kelvins.
Curveball Question #4 – Explain if the enthalpy change due to neutralisation involving weak acids and weak bases will be larger, smaller or equal to that of a strong acid-strong base neutralisation.
Here is an alternative wording to the same question above to make it more clear for you. Some examples of neutralisation involving weak acids and bases are weak acid reacting with strong base, weak base reacting with strong acid or weak acid reacting with weak acid. Explain whether or not enthalpy change due to neutralisation be larger, smaller or equal than a neutralisation reaction involving a strong base with a strong acid.
Curveball Question #5 – Construct a mathematical expression that you can use in order to calculate the deviation of your experimental value for the enthalpy change due to neutralisation from the published value
Curveball Question #6 – Suppose that you poured water (distilled) into calorimeter which you measured to have an initial temperature of 24 degrees celsius. A fellow chemist group was playing an ice cube and an ice cube accidentally flew into your calorimeter, LOL! Assuming that the ice cube that entered your calorimeter had an initial temperature of zero degrees celsius, calculate the enthalpy change due to fusion of the ice cube with the water. Suppose that the initial temperature of the solution decreased from 24 degrees celsius down to 16 degrees celsius after the fusion of the ice cube and water was complete and stabilised. Assume that the mass of ice cube is 1 gram and mass of water is 100g.
Curveball Question #7 – Outline two safety precautions that you need to take when performing calorimetry experiments and suggest ways to minimise them.
Curveball Question #8 – Suppose you are diluting a strong acid, explain the reason to why you would small quantities of the strong acid to water and not the other way around, i.e. why you should not add water to strong acid to dilute the acid?
Week 5 Extension Questions
NOTE: Extension Questions are extra exam-style questions that will be posted throughout the HSC year.
Extension Questions and Solutions will be posted more regularly once the complete set of notes for Module 5, 6, 7 and 8 are complete before the end of Term 1 2019.
Solutions to Week 5 Homework Questions
Solution to Question #1
Edit to solution to Question #1:
The balance equation should be H2SO4 + 2KOH -> K2SO4 + 2H2O
Since mole ratio between H2SO4 : KOH is 1:2, this would mean that 1 mole of KOH should be reacted for every 0.5 mol of sulfuric acid that is consumed.
Since we have 1 mole of KOH to begin with (as stated in question), there is no limiting reagent.
So, 0.5 mol of water is still formed. The enthlapy of neutralisation is correct in the answer above.
Solution to Homework Question #4: (This is the last solution that was written up for this Module)